Gold in Quartz - How to Assess the Weight of Gold in a Specimen
We have a had a look for methods of assessing the weight of gold in quartz or another specimen rock and found the explanations a bit complicated.
We like to simplify things and make it easy for you so on this page you can just download a spreadsheet to enter a few numbers and you will get a reasonable assessment of the weight of gold in quartz or ironstone or whatever other materials your gold nugget is encased in.
This video will help you understand a few different methods to assess the weight of Gold in a Quartz Specimen;
Specific Gravity
Just a refresher on specific gravity extracted from the Gold Facts page;
Specific gravity is a measure of relative density - the ratio between the weight of a substance and weight of an equal volume of water at 4 degrees C (39 degrees F) - The Specific Gravity of Water at 4 Deg C is 1.0. The specific gravity of gold is around 19.3
The SG of water at this temperature is 1.0 and it's WEIGHT is assumed as 1 gram per millilitre or 1kg per litre.
An Explanation of Specific Gravity and the Formula to Calculate it.
....If you are really interested, that is!
To explain that in other words, the specific gravity of gold is 19.3 which means the weight of gold is 19.3 times that of an equivalent volume of water (at 4 degrees C).
If water weight is 1 gram per ml then Gold at an SG of 19.3 is 19.3 times as dense as water or 19.3 grams
In the case of quartz which has a specific gravity of 2.65 the weight of the quartz is 2.65 times that of an equivalent volume of water.
Methods
Remember that these methods will only give you a ESTIMATE of the gold content, be cautious with the results, the only true way is to process the specimen and recover the gold.
You may not want to do that however, particularly if you have a beautiful specimen that you would like to keep.
Here are a few methods that you can use to get an estimate of the gold content of a specimen rock or gold in quartz
Specific Gravity Water/Air Calculation - Method 1
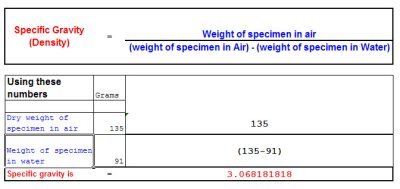
The formula for calculating the specific gravity or Density of a Specimen rock
However the specimen that you want to estimate the gold content in also contains other material such as quartz, quartzite or ironstone or other substances so this needs to be taken into consideration in the calculation.
Excel Spreadsheet for the calculation
The spreadsheet described in the video above (Method 1) can be downloaded here Spreadsheet for the calculation of Specific Gravity.
What you need for this method is a set of accurate scales either digital or beam balance that you can weigh your specimen at an accuracy of 0.1grams with capacity to weigh up to the size of your specimen.
You will also need a method of suspending the rock specimen in water so you can obtain its wet weight.
We have just made a rough bridge to suspend the specimen and that is shown in the video.
The basic process;
- Weigh the specimen while it is dry,
- Work out the weight of the specimen while it is suspended in water which is the Wet Weight.
- Enter the main rock material of the specimen into the spreadsheet (quartz, ironstone, other)
- Enter the dry and wet weight of the specimen into the spreadsheet.
Then the spreadsheet will calculate a rough estimate of the gold content.
Important points to remember;
- Accuracy is crucial, particularly assessing the wet weight of the specimen!
- When you are weighing the specimen suspended in water it must not touch the bottom or side of the container
- The estimate of the gold content is just that, an estimate. It is NOT exact.
Another Simpler Method to Calculate the Weight of Gold In Quartz
This method is also explained on the video at method 2.
What you need is;
- A Set of Accurate Scales
- A container for water that is big enough to take the rock you are weighing without spilling the contents.
- A marker to mark the water container at the different water levels required.
The steps are -
- Weigh the rock and record the dry weight.
- Partially fill the water container with water and mark the level at the top.
- Place the rock specimen in the container.
- Mark the new level of the water on the side of the container.
- Remove the rock and make sure all the water droplets are returned to the container.
- Zero or tare the weight on the scales with the water container on it back to zero.
- Fill the container up to the new level and record the weight of the water replaced.
- Multiply that weight by the SG of the main matrix of the material. If it is quartz multiply it by the SG of Quartz 2.65.
- Take that weight away from the dry weight of the rock and the weight left is a rough estimate of the gold in that specimen.
Remember, this is definitely not as accurate as the first method and both methods are only rough estimates - be cautious with their use.
Table for Weight of Gold in Quartz
If you have calculated the Specific Gravity of your gold rock and the material enclosing the gold is mainly quartz then a quick and dirty method is to calculate the rough % of gold is using a table;
Gold in Quartz Table to easily find the approximate percentage of your rock specimen which is gold
Another Displacement Method to Calculate the Weight of Gold In Other Rocks
Rex Whitehead of Mt Isa sent this to us. Thanks Rex.
The method I have used, is by water displacement, marking the side of a transparent container as you do in one of your methods. I have been using graduated laboratory beakers. The smaller the beaker, the greater the accuracy.
What I do is first weigh the dry specimen.
Place sufficient water in the beaker, that will cover the specimen. Mark the water level accordingly.
Then place the specimen in the water.
Mark the new water level, with the specimen in it.
I then tip the water and specimen out, and refill the beaker to the original level, to what it was, prior to placing the specimen in it. I use a pipette or syringe to get the water back to the level accurately.
I then place rocks I have collected, that are of the same material as the host rock, (whether it be quartz or ironstone etc.), in the beaker until the water level comes up to the level of what it was when the specimen was in the water.
You will need a collection of different sized rocks to make this level up exactly. I then remove these rocks, thoroughly dry them, then weigh them. The difference in weight to the Dry Weight of the specimen, should be the gold content in the specimen.
Return to Gold Prospecting home Page from Gold In Quartz page
![[Most Recent Gold Price Quotes in Australian $]](https://www.kitconet.com/charts/metals/gold/t24_au_en_auoz_2.gif)
![[Most Recent Gold Price Quotes in US $]](https://www.kitconet.com/charts/metals/gold/t24_au_en_usoz_2.gif)